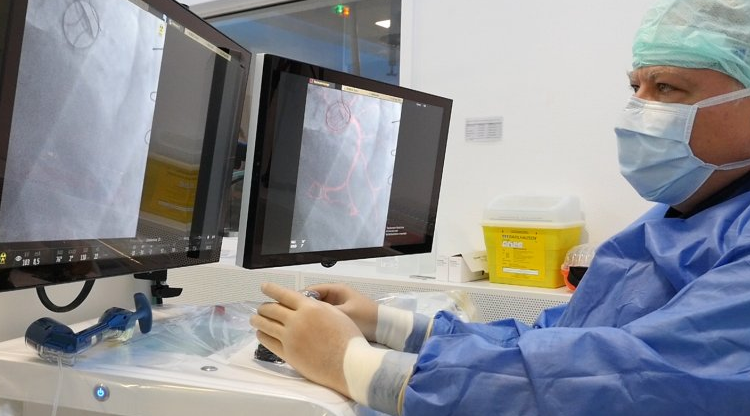
GE HealthCare Receives FDA Clearance of Allia IGS Pulse—the Next Generation of Image-Guided Systems Designed for Cardiac Imaging Excellence
The newest addition to the company’s line of image guided systems (IGS), Allia IGS Pulse, was just given US FDA 510(k) clearance, according to GE HealthCare. Regardless of patient size, Allia IGS Pulse’s novel imaging chain is built to deliver outstanding imaging at the optimal dose for noticeable impact in difficult cardiac treatments.
Cardiovascular disease (CVD) is now the world’s largest cause of mortality, and its prevalence is continuing to rise in the context of contemporary healthcare. Cardiology techniques continue to advance as the need for less invasive surgery rises and practitioners attempt to treat CVDs. By streamlining processes and offering great picture quality to support clinicians in delivering high-quality treatment, GE HealthCare is committed to assisting interventional cardiologists, vascular surgeons, and other interventional experts use image-guiding technologies to their full potential.